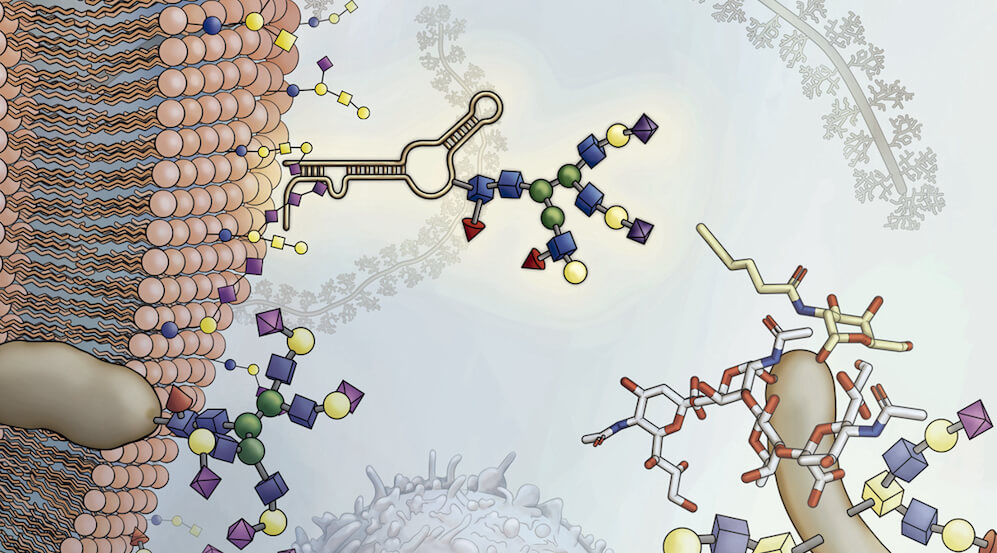
Cells in our body bristle with sugars known as glycans that other cells can recognize via specialized receptors. By attaching to and modifying proteins and fats, glycans influence how proteins fold, how cues are sent between cells, and other cell-to-cell interactions. As just one example of glycans’ importance, our blood types (A, B, O) depend on which sugars red blood cells incorporate into their protective coating.
New research led by Ryan Flynn, assistant professor of stem cell and regenerative biology at Harvard, reveals a whole new universe of sugar-coated molecules that attach to our cells. Called glycosylated RNAs or glycoRNAs, they are surprisingly pervasive — yet nobody had previously done the right experiments to find them. They open up another dimension for exploring how our bodies work and the origins of disease.
Flynn discovered glycoRNAs while at Stanford University in the lab of Carolyn Bertozzi. And now, in his new lab at Boston Children’s Hospital, he’s begun exploring these molecules’ importance. In a study recently published in Cell, he and Bertozzi fleshed out some early details.
“We found that glycoRNAs are on the cell surface, just like proteins and lipids,” said Flynn. “This is exciting because it means that glycoRNAs can participate directly in cell-to-cell communication. That was previously thought to be off limits for RNAs, which had not been thought to play a role on the cell surface.”
Exploring a new biological actor
Flynn and Bertozzi revealed that glycoRNAs consist of small, noncoding RNAs — which are known to play regulatory roles inside cells — coated with a class of sugars known as N-glycans. When investigating the purpose of glycoRNAs, Flynn and Bertozzi noticed that some of the molecules’ N-glycans were capped by a sugar called sialic acid. This inspired them to investigate whether glycoRNAs interacted with Siglecs, certain receptors on immune cells known to bind to sialic acid.
“This was where the hair on our neck stood up,” Bertozzi said.
Certain members of the Siglec family, a class of proteins that regulate immune cells, bound cells when RNA was present. This led Flynn to suspect that glycoRNAs could help fine-tune our immune system, and his new lab will investigate glycoRNAs’ potential role in autoimmune disease.
“Ryan is starting his independent career sitting on a bombshell,” Bertozzi said.
Bridging two fields
The textbook-changing discovery of glycoRNAs was fortuitous. Flynn, who was trained in RNA biology, joined Bertozzi’s lab, which focuses on glycoscience, to better understand how glycans work. Bertozzi and others have for decades used tools from bioorthogonal chemistry, a field Bertozzi’s lab pioneered, to examine protein and lipid glycans on the cell surface.
“At the time, there was no intersection known between the RNA world and the glycan world. They were two separate areas of biology,” Bertozzi said.
Flynn became fascinated with glycans, and paired Bertozzi’s tools with methods that detect RNAs. “There were two spheres of information I joined together, initially with not much to go on,” he said.
Flynn began by labeling RNAs and glycans with special probes. To his surprise, the glycan probes kept turning up with RNAs — albeit very tiny RNAs, among the smallest that exist. He and Bertozzi thought at first there must be an artifact or contaminant in their samples. But Flynn confirmed the finding through a battery of experiments.
Once he proved that glycoRNAs are real, Flynn next showed that glycoRNAs are concentrated at the cell surface, just like glycoproteins and glycolipids — and ready to interact with other cells. He now wants to figure out how RNAs and glycans are able to hook up, since they traditionally inhabit different compartments of the cell.
“Everyone thought we had two ‘hands’ on the cell surface, and now we know we have three,” Flynn said. “I used every single lever of RNA biology that I had, but the discovery of glycoRNAs would not have been possible had I not been in Carolyn’s lab.”
The original version of this story was published on the Boston Children’s Hospital website on May 17, 2021, under the title, “When worlds collide: Glycosylated RNAs upend cell biology as we know it.”
The illustration was originally published on the Howard Hughes Medical Institute website.
Source article: Flynn, R. A., et al. (2021). Small RNAs are modified with N-glycans and displayed on the surface of living cells. Cell. DOI: 10.1016/j.cell.2021.04.023
This work was supported by the Damon Runyon Cancer Research, Burroughs Wellcome Fund Career Award for Medical Scientists, National Institutes of Health (R01 CA200423-17, R01 AI141970, F30-CA232541), Stanford Medical Scientist Training Program, National Institute of General Medical Sciences (F32-GM126663-01), National Science Foundation Graduate Research Fellowship (DGE-114747), and NSF-GRF/Stanford Graduate Fellowship/Stanford ChEM-H Chemistry/Biology Interface Predoctoral Training Program.
